Analysis and purification of biomolecules, such as nucleic acids, peptides, and proteins
YMC provides a wide range of bioseparation products for use in ion exchange, size exclusion, hydrophobic, reversed-phase separations designed for peptides, proteins, nucleic acids, sugar chains, and viral vectors. We provide bulk packing materials (media) for large scale production as well as columns for analysis.
Our product range includes chromatography systems for preparative purification from a laboratory-scale to a plant-scale. With our bulk packing materials (media), we provide total support services from separation method development to preparative isolation.
We can also offer custom purification services. With our in-house manufactured packing materials, columns, and systems, we can provide highly efficient preparative purification according to your needs.
Accura BioPro IEX is an ion exchange column ideal for analysis of biomolecules. It shows excellent peak shapes of sensitive compounds and no carry over.
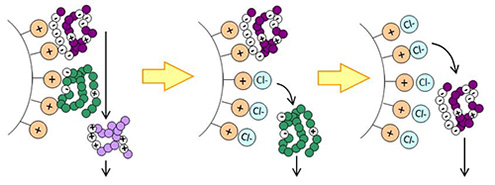
BioPro IEX SmartSep and BioPro IEX media are ion exchange media with high dynamic binding capacities which enable high productivity. These are suitable for separation of biomolecules, such as proteins, antibodies and oligonucleotides.
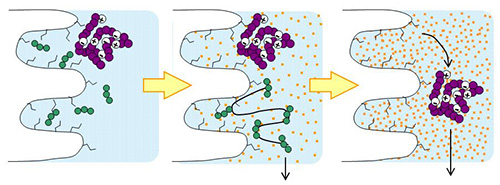
YMC-Triart columns with organic/inorganic hybrid silica are extremely durable even at high temperatures. Therefore it is useful for separation of biomolecules such as peptides, proteins, antibodies, and oligonucleotides. YMC-Triart columns are also suitable for LC/MS analysis because it gives excellent peak shape even with mobile phase containing formic acid. For the analysis of compounds that are sensitive to adsorption on the metal surfaces of the column, the bioinert coated column Accura Triart is suitable.
YMC-Triart Prep, the bulk packing materials for purification, are also available.
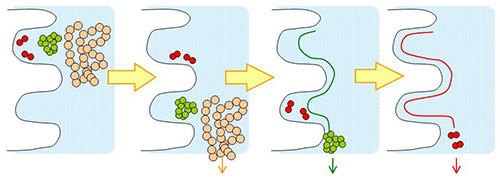
YMC-SEC MAB and YMC-Pack Diol are silica-based aqueous size exclusion (GFC; gel filtration chromatography) columns. YMC-SEC MAB is optimized for separation of antibodies. YMC-Pack Diol columns can be selected four different pore sizes depending on the molecular weight rage.
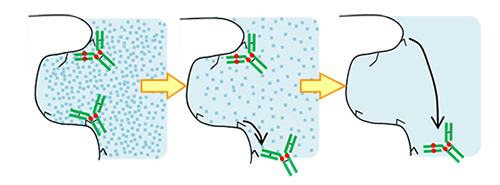
BioPro HIC is effective for analysis of antibody-drug conjugates (ADCs), and also useful for separation of biomolecules that is difficult to be separated by IEX and affinity chromatography. It can perform fast analysis with high resolution and low operating pressure.
The low pressure preparative system, BioStream, is a biochromatography system that is suitable for ion exchange and size exclusion chromatography which is capable of a wide range of purification scales. With YMC Pilot made of glass or acrylic plastic, we offer a chromatography system for various purposes from R&D to GMP plant applications.
The automated preparative system, K-Prep, is most suited for reversed phase applications. It can be applied to laboratory scale through to plant scale, especially when used with automatic self-packing, dynamic axial compression columns. Customization is available for your needs including GMP validation and explosion proof operation.
Our API Purification Plant, which is compliant with GMP requirements, can provide a wide range of chromatography purification requirements from laboratory scale to plant scale.