Selection guide for biochromatography modes
YMC products for separation of biomolecules such as oligonucleotides, peptides and proteins are categorized into four chromatography modes (ion exchange, size exclusion, hydrophobic interaction and reversed-phase).
Ion exchange chromatography is routinely used for charge variant analysis of biopharmaceuticals, and also be effective for separation of oligonucleotides. Hydrophobic interaction chromatography allows biomolecules to be separated on the basis of hydrophobicity even under non-denaturing conditions. Reversed-phase chromatography offers a rapid and high resolution analysis for biomolecule characterization such as peptide mapping. Size exclusion chromatography is suitable for the analysis of aggregates and fragments in biopharmaceuticals.
Selection of the appropriate chromatography mode according to separation purposes is important to perform the method development studies.
| Separation mode | Ion exchange | Size exclusion | Hydrophobic interaction | Reversed-phase |
|---|---|---|---|---|
| YMC products | Accura BioPro IEX BioPro IEX Column |
YMC-SEC MAB YMC-Pack Diol |
BioPro HIC | YMC-Triart (for biochromatography) |
| Key factor | Electrostatic charge | Molecular size | Hydrophobicity | Hydrophobicity |
| Usable molecular weight range | Up to several millions | Up to about 1,000,000 | Up to several millions | Up to about 150,000 |
Resolution |
+++ | ++ | +++ | +++ |
| Throughput | ++ ~ +++ | + | +++ | +++ |
| Loading capacity | +++ | ++ | +++ | ++ |
| Sample stability | +++ | +++ | +++ | + ~ ++ |
| Typical applications | Charge variant analysis of biopharmaceuticals | Analysis of aggregates and fragments in biopharmaceuticals | DAR analysis of antibody-drug conjugates | Characteristic analysis coupled with mass spectrometry (Ex: peptide mapping) |
Ion exchange chromatography is a technique used to separate ionic molecules by differences in their net charge. Generally, resins (media), which have cationic or anionic groups, are used as stationary phases and the counter-ion added buffers are used as mobile phases.
In the sample application step, molecules with opposite charge to the media bind to them by ionic interaction. Next, in the elution step, by increasing the concentration of the counter-ions in the mobile phase, molecules with the lowest net charge are eluted first and those with higher charge are eluted later.
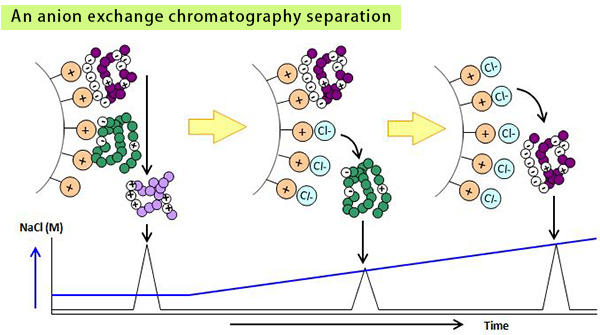
Charge variants of proteins are usually analyzed by ion exchange chromatography.
Monoclonal antibodies were separated by using BioPro IEX SF in salt gradient mode and in pH gradient mode. Sharp peaks of charge variants and isoforms were obtained in both modes.
| 1. natalizumab |
| 2. cetuximab |
| 3. adalimumab |
| 4. denosumab |
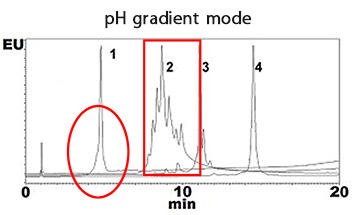
| Column | BioPro IEX SF (5 µm), 100 X 4.6 mmI.D. |
|---|---|
| Eluent | A) CX-1 pH Gradient Buffer A* (pH 5.6) B) CX-1 pH Gradient Buffer B* (pH 10.2) 0-100%B (0-20 min) |
| Flow rate | 0.6 mL/min |
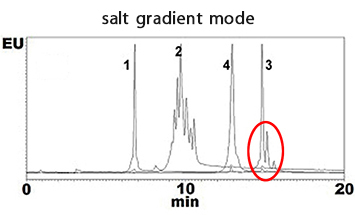
| Column | BioPro IEX SF (5 µm), 100 X 4.6 mmI.D. |
|---|---|
| Eluent | A) 10 mM MES-NaOH (pH 5.7) B) 10 mM MES-NaOH (pH 5.7) containing 1 M NaCl 0-20%B (0-20 min) |
| Flow rate | 0.6 mL/min |
Size exclusion chromatography is a technique used to separate molecules by their size. As a stationary phase, silica gels and polymers which have pores with a network structure are used and as a mobile phase, buffers which have high solubility and pH stability towards the compounds are used. Smaller molecules that can penetrate deeper into the pores are eluted later from the column while larger molecules which cannot enter the pores are eluted earlier from the column.
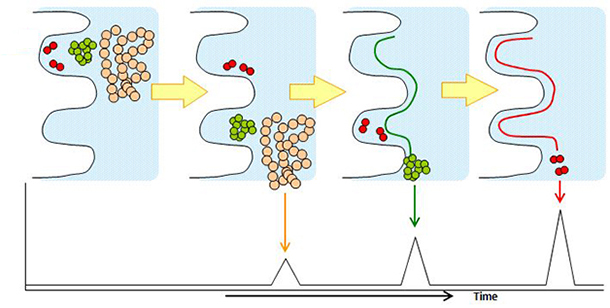
Size exclusion chromatography is used for separation of monomer, dimer, aggregates and fragments of proteins.
Humanized monoclonal antibody was analyzed using YMC-SEC MAB. The chromatogram shows excellent peak shape, and good separation between aggregates and a monomer peak. Additionally, a monoclonal antibody digested by papain and an intact monoclonal antibody were analyzed using YMC-SEC MAB column. Since the column shows excellent separation between Fc and Fab fragments, which have similar amount of MW, it is effective for separation of antibody fragments.
Analysis of monoclonal antibody aggregates 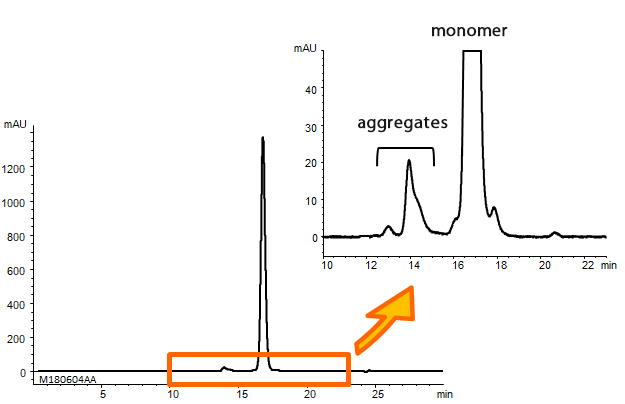
| Column | YMC-SEC MAB (3 µm, 250 Å) 300 X 4.6 mmI.D. |
|---|---|
| Eluent | 0.1 M KH2PO4-K2HPO4 (pH 7.0) containing 0.2 M NaCl |
| Flow rate | 0.165 mL/min |
| Temperature | 25°C |
| Detection | UV at 280 nm |
| Injection | 10 µL (5 mg/mL) |
| Sample | Humanized monoclonal antibody |
Analysis of monoclonal antibody fragments
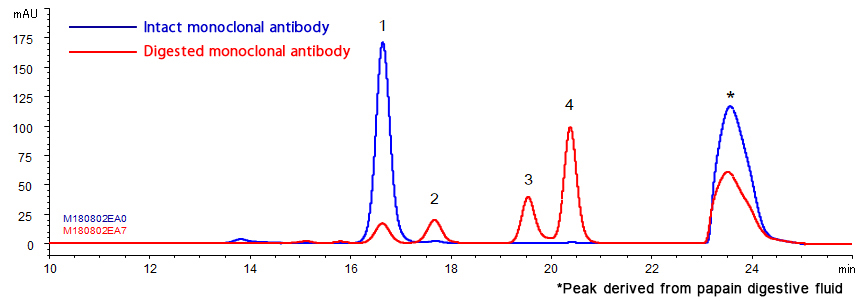
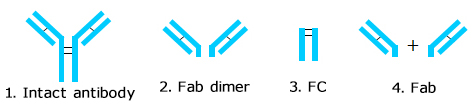
| Column | YMC-SEC MAB (3 µm, 250 Å), 300 X 4.6 mmI.D. |
|---|---|
| Eluent | 0.1 M KH2PO4-K2HPO4 (pH 7.0) containing 0.2 M NaCl |
| Flow rate | 0.165 mL/min |
| Temperature | 25°C |
| Detection | UV at 280 nm |
| Injection | 2 µL (3 mg/ml) |
| Sample | Humanized monoclonal IgG1 + papain digestive fluid |
Hydrophobic interaction chromatography utilizes a high salt concentration of mobile phase to initiate the hydrophobic interaction between the stationary phase and biomolecules. Decreasing the salt concentration of mobile phase weakens the hydrophobic interaction, and elutes the biomolecules in ascending order of hydrophobicity.
Unlike reversed-phase chromatography, hydrophobic interaction chromatography allows biomolecule separation without organic solvents inducing the denaturation of proteins.
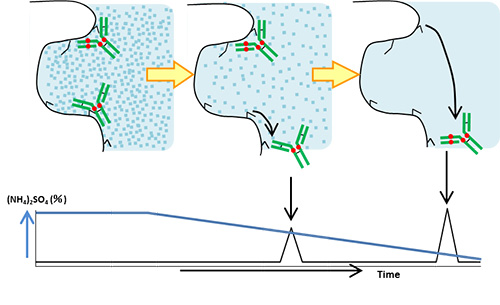
Hydrophobic interaction chromatography is used for separation of antibody-drug conjugates (ADCs) composed of monoclonal antibodies linked to small molecular drugs (payloads) because of the hydrophobicity heterogeneity of ADCs based on the drug-to-antibody ratio (DAR).
BioPro HIC HT showed good separation in the DAR analysis of ADCs.
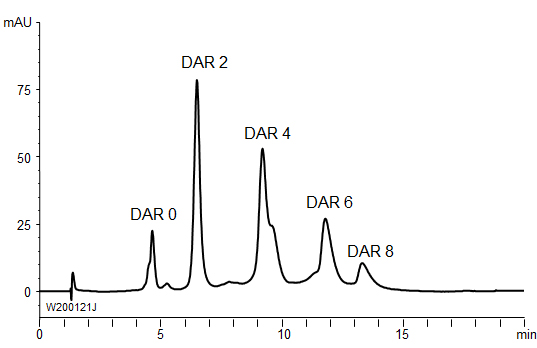
| Column | BioPro HIC HT (2.3 µm), 100 X 4.6 mmI.D. |
|---|---|
| Eluent | A) 20 mM NaH2PO4-Na2HPO4 (pH 7.0) containing 1.0 M (NH4)2SO4 B) 20 mM NaH2PO4-Na2HPO4 (pH 7.0)/2-propanol (90/10) 0-100%B (0-15 min), 100%B (15-20 min) |
| Flow rate | 0.5 mL/min |
| Temperature | 25°C |
| Detection | UV at 280 nm |
| Injection | 10 µL |
| Sample | Brentuximab vedotin (2.5 mg/mL) |
Reversed-phase chromatography is a technique used to separate molecules by hydrophobic interactions between compounds and a stationary phase and between compounds and a mobile phase. Stationary phases include silica gels and hybrid silica to which hydrophobic functional groups such as C18, C8, and C4 are bonded are used, with mobile phases being mixtures of organic solvents and aqueous acid solutions or buffers. As the concentration of organic solvents in the mobile phase is increased, the molecules with lower hydrophobicity are eluted first.
Reversed-phase chromatography is used for analysis of antibody fragments and peptide mapping.
Below are the chromatograms of monoclonal antibody fragments analysis with mobile phase containing either TFA or formic acid.
YMC-Triart achieves excellent peak shape even when using mobile phase containing formic acid. Therefore it is ideal for the high sensitive LC-MS analysis.
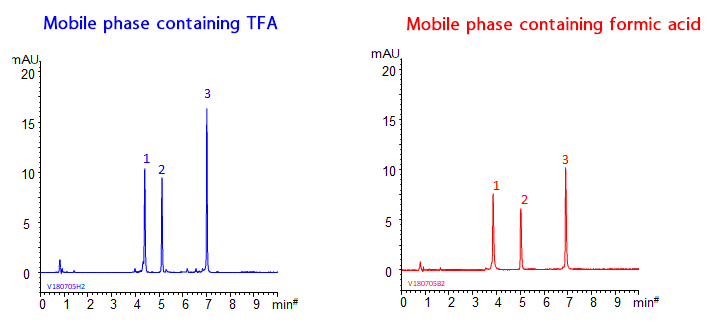
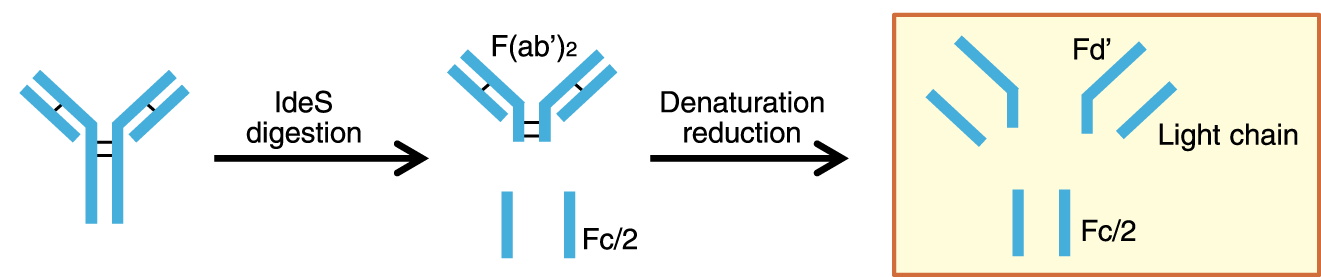
| Column | YMC-Triart Bio C4 (1.9 µm, 300 Å), 150 X 2.1 mmI.D |
|---|---|
| Eluent <TFA> | A) water/TFA (100/0.1) B) acetonitrile/TFA (100/0.1) 25-50%B (0-10 min), 90%B (10-12.5 min) |
| Eluent <Formic acid> | A) water/formic acid (100/0.1) B) acetonitrile/formic acid (100/0.1) 20-45%B (0-10 min), 90%B (10-12.5 min) |
| Flow rate | 0.4 mL/min |
| Temperature | 80°C |
| Detection | UV at 280 nm |
| Injection | 4 µL (0.25 mg/mL) |