
Preparative purification was performed to obtain more than 200 mg of degradation product in total amount with over 95% purity for structural determination of the pharmaceutical degradation compound.
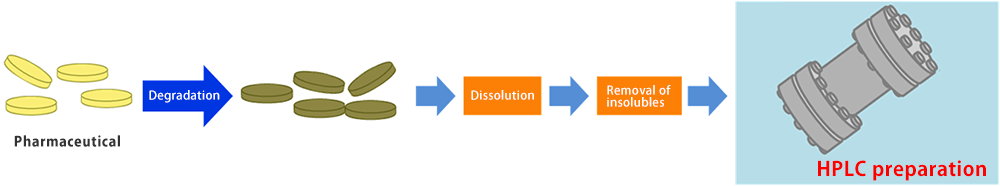
Separation method was developed on the analytical scale. Mobile phase pH was optimized in order to completely separate the target compound from the rest of the compounds. By applying the conditions for high resolution, loadability of a single run can be increased.
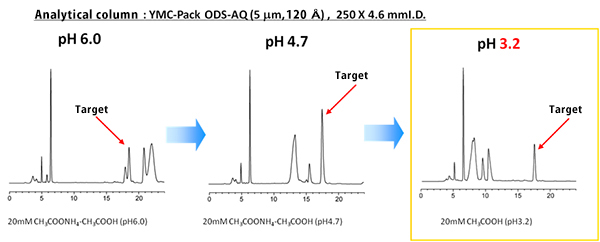
| Eluent | Buffer/acetonitrile (85/15) |
|---|---|
| Flow rate | 0.8 mL/min |
| Temperature | 35℃ |
| Detection | UV at 270 nm |
Separation conditions were studied at room temperature because the heated condition is not preferable on the scaled up purification. Even at room temperature, the target compound and the rest of the compounds were well separated and kept good peak shapes.
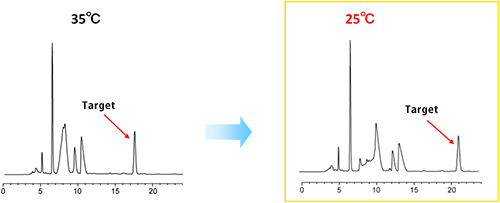
| Eluent | 20 mM CH3COOH (pH 3.2) /acetonitrile (85/15) |
|---|---|
| Flow rate | 0.8 mL/min |
| Detection | UV at 270 nm |
| Injection | 20 μL |
Based on the conditions studied on the analytical scale, column size and particle size were chosen. After the lab-scale preparation study, the large-scale preparation was performed.
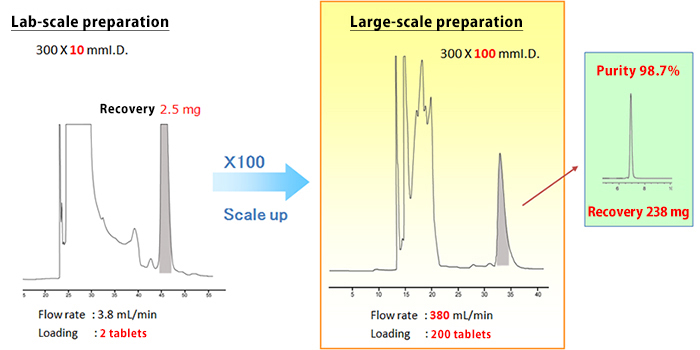
| Column | YMC*GEL ODS-AQ (15 µm, 120 Å) |
|---|---|
| Eluent | 20 mM CH3COOH/acetonitrile (85/15) |
| Temperature | ambient |
| Detection | UV at 270 nm |