According to pharmacopoeias, many drugs are prescribed to be analyzed by high pressure liquid chromatography (HPLC) using conventional column dimensions and parameters. The United States Pharmacopeia (USP) and the International Harmonization of Pharmacopoeias allow for changes in LC parameters to the extent that they fulfill system suitability requirements for UHPLC.
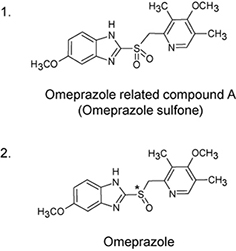
The LC analyses for Esomeprazole Magnesium [ORGANIC IMPURITIES], listed in USP 42, were performed under the USP-compliant and time-saving conditions. YMC-Triart C8, organic/inorganic hybrid silica column categorized as packing L7, with particle size of 5 µm and column dimensions of 150 X 4.6 mm I.D. was used in the USP-compliant condition. The time-saving analyses were performed with particle size of 3 µm and 1.9 µm. These column dimensions follow the range of L/dp (L: column length, dp: particle size) permitted by the International Harmonization of Pharmacopoeias (22,500 ≤ L/dp ≤ 45,000).
Both the USP-compliant and time-saving conditions met the system suitability requirement.
YMC-Triart columns exhibit the same selectivity across different particle sizes, allowing for easy method transfer from HPLC to UHPLC as shown here.
| USP Monograph | USP-compliant | Time-saving (HPLC) | Time-saving (UHPLC) | |
|---|---|---|---|---|
| Column | 5 µm, packing L7, 125 X 4.0 or 150 X 4.6 mmI.D. |
YMC-Triart C8, 5 µm, 150 X 4.6 mmI.D. (L/dp = 30,000) |
YMC-Triart C8, 3 µm, 100 X 3.0 mmI.D. (L/dp = 33,300) |
YMC-Triart C8, 1.9 µm, 50 X 2.0 mmI.D. (L/dp = 26,300) |
| Eluent | solution A*1/acetonitrile(29/11) *1Dissolve 0.725 g of NaH2PO4・2H2O and 4.472 g of Na2HPO4 in 300 mL of water, and dilute with water to 1000 mL. Dilute 250 mL of this solution with water to 1000 mL. If necessary, adjust with H3PO4 to a pH of 7.6. |
|||
| Flow rate | 0.8-1 mL/min | 1.0 mL/min | 0.7 mL/min | 0.5 mL/min |
| Temperature | No description | 45°C | ||
| Detection | UV at 280 nm | |||
| Injection volume | 50 µL | 20 µL*2 | 6 µL | 1 µL |
| System suitability requirement | Rs (1, 2) ≥3.0 | |||
*2Maximum injection volume of the equipment used
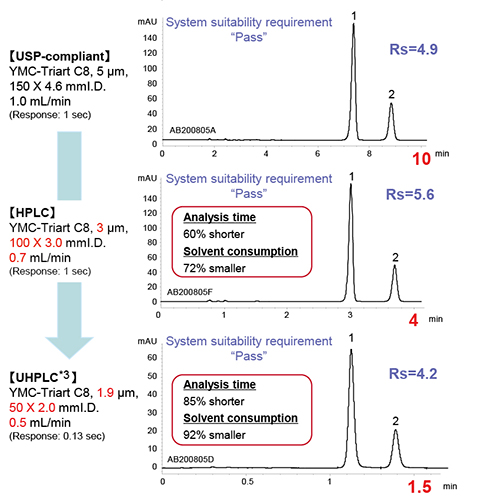
*3Optimized instrument dead volume and data acquisition speed.