Analysis of ionic compounds by reversed-phase HPLC is conducted with the pH of eluent controlled using acid or buffering agent. However, a separation with a pH range which is not optimum for the compound of interest could cause problems such as double peak or peak broadening. Even if the peak shape is satisfactory, retention time reproducibility could in some cases not be obtained.
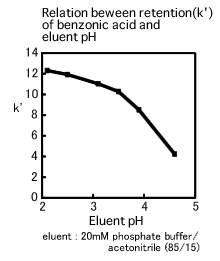
The relation between retention of benzonic acid and pH value is shown in the figure below. Although the k' falls within relatively narrow limits in the region where the pH ranges from 2 to 3.5, it varies widely in the region where the pH ranges from 3.5 to 4.5. The pKa of benzonic acid is 4.2 and it is noticeable that the region where the k' most widely varies is near the pKa. If the eluent pH is adjusted to the region near the pKa with the wide variation of the k', the result might not be reproducible since the slight error of the pH adjustment could be of great impact on separation. In fact, the eluent pH variation of just 0.1 significantly affects separation. Consequently, it is desirable that the eluent pH should be more than 1 off the pKa. If the pKa is unknown, the eluent pH should be adjusted to within the region where the impact on separation seems minimal, after having deliberately considered the relation between the eluent pH and the retention time.
When considering the pH value, it is also important to confirm the influence on separation using several eluents with their pHs adjusted to be slightly different from each other.