Supports
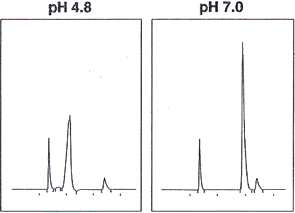
Analysis of ionic compounds by reversed-phase HPLC requires the pH control of eluent with acid or buffering agent. The pH control stabilizes the dissociation state of compounds and enhances the reproducibility of retention and separation. Unsuitable pH in analysis causes problems such as a double peak or peak broadening. As shown in the figure below, an identical compound is eluted showing the double peak in pH4.8 eluent, while showing a sharp peak in pH7.0. When analyzing an ionic compound, finding the right pH range for each functional group will result in avoiding such problems.