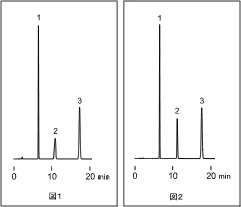
Although a column is apt to be thought of as a cause of HPLC analysis not showing proper data trace, many cases are attributed to other causes than a column which include improper maintenance operations. This article discusses the case in which the grade of a solvent has impact on peak shapes. Here is a chromatogram of the basic compound analysis with eluent of acetonitrile/ water. Peak 2 represents the basic compound.
Figures below show chromatogram of two operations conducted under identical conditions except that the acetonitrile used was of different grades. One was HPLC grade (Figure 1); the other was reagent grade (Figure 2). While the peak shape was broadened with HPLC grade acetoniteile, it was much improved when using reagent grade. The peak shape differences were observed depending on acetonitrile products of different makers even though they were of the same special grade. This may be because traces of impurities contained in acetonitrile behave in the same way as modifier added to an eluent.
Replacing eluent with acetonitrile/ 5mM ammonium acetate produced a chromatogram like that in Figure 2 either with reagent or HPLC grade acetonitrile.
To avoid the influence of different grades, solvent specialized for HPLC must be used. Even compounds which have dissociation groups can be analyzed with eluent containing no acid or salt, though eluents with additives such as salt must be used when reproducibility is important.