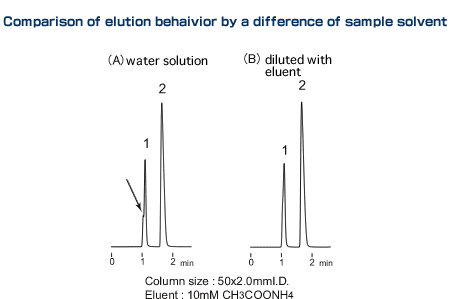
Although dissolving sample compounds in the eluent is the basic procedure of HPLC, that is not possible with some samples due to their solubility or the sample preparation method required. Then, excessive differences in solvent strength or pH between the eluent and the sample solvent could cause such problems as double peaks and broadened peaks. The cause of these phenomena is considered to be dispersion or a varying degree of dissociation of the analyte resulting from the sample solvent being temporarily replaced by the eluent in the column. Examples of solutions with this type of problem are discussed below. The chromatogram (A) represents 2µL of water solution of a sample injected, and a shoulder is observed. As the pHs were different between the sample solution and the eluent, 4µL of this sample was injected after diluting it by 2 times with the eluent to reduce the pH difference.The peak shape was much improved as in chromatogram (B).
Other solutions of this type of problems include to reduce the sample volume and to dilute the sample solution with the low strength solvent. If peak deterioration is observed, you should confirm the sample solution has the identical characteristics as the eluent. If otherwise, you should consider the solutions discussed above.